
 19:12
19:12
2025-09-11 14:41

 1:42:59
1:42:59

 1:42:59
1:42:59
2025-05-03 23:00

 2:15
2:15

 2:15
2:15
2025-09-25 22:19

 7:49
7:49

 7:49
7:49
2024-04-24 10:16

 10:19
10:19

 10:19
10:19
2024-11-03 20:46

 14:34
14:34

 14:34
14:34
2024-09-25 14:43

 5:04
5:04

 5:04
5:04
2024-04-21 07:00

 1:23:24
1:23:24

 1:23:24
1:23:24
2025-09-18 12:00

 24:23
24:23

 24:23
24:23
2025-09-11 09:20

 34:56
34:56

 34:56
34:56
2025-09-12 16:44

 0:36
0:36

 0:36
0:36
2025-09-26 18:00
 8:02
8:02

 8:02
8:02
2023-08-09 09:10

 3:43
3:43

 3:43
3:43
2023-01-30 16:51

 10:37
10:37

 10:37
10:37
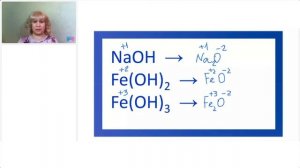
Классы НЕОРГАНИЧЕСКИХ веществ | Как давать НАЗВАНИЯ ВЕЩЕСТВАМ в химии |Кислоты основания соли оксиды
2022-03-12 17:52

 32:16
32:16

 32:16
32:16
2025-09-20 09:34

 7:40
7:40

 7:40
7:40
2025-09-25 17:00

 1:50:16
1:50:16

 1:50:16
1:50:16
2025-09-15 14:19

 4:18
4:18
![Roza Zərgərli, Мурад Байкаев - Неизбежная любовь (Премьера клипа 2025)]() 2:34
2:34
![EDGAR - Мой брат (Премьера клипа 2025)]() 3:33
3:33
![Анжелика Агурбаш - Утро (Премьера клипа 2025)]() 3:33
3:33
![Илёс Юнусий - Каранг она якинларим (Премьера клипа 2025)]() 3:36
3:36
![Cvetocek7 - Запретила (Премьера клипа 2025)]() 2:49
2:49
![Бобур Ахмад - Куролмаслар (Премьера клипа 2025)]() 3:33
3:33
![KAYA - Девочки, отмена (Премьера клипа 2025)]() 3:53
3:53
![Bruno Mars ft. Ed Sheeran – Home to You (Official Video 2025)]() 3:25
3:25
![Мохито, DJ DimixeR - Перед рассветом (Премьера клипа 2025)]() 2:29
2:29
![Тахмина Умалатова - Не потеряй (Премьера клипа 2025)]() 4:10
4:10
![Жамхур Хайруллаев - Битта дона (Премьера клипа 2025)]() 2:49
2:49
![Бриджит - Ласковый май (Премьера клипа 2025)]() 3:20
3:20
![Наталья Влади - Я обещаю (Премьера клипа 2025)]() 3:00
3:00
![Мухит Бобоев - Маликам (Премьера клипа 2025)]() 3:18
3:18
![Зара - Прерванный полет (Премьера клипа 2025)]() 5:08
5:08
![Соня Белькевич, КРЕСТОВ - Малиновый закат (Премьера клипа 2025)]() 3:24
3:24
![Амина Магомедова - Не пара (Премьера 2025)]() 3:40
3:40
![Евгений Коновалов - Зачем ты меня целовала (Премьера клипа 2025)]() 3:17
3:17
![Tamo ft Djan Edmonte - Ну что красавица (Премьера клипа 2025)]() 3:10
3:10
![Антон Макарский - Не уходи (Премьера клипа 2025)]() 3:41
3:41
![Крысы: Ведьмачья история | The Rats: A Witcher Tale (2025)]() 1:23:01
1:23:01
![Крушащая машина | The Smashing Machine (2025)]() 2:03:12
2:03:12
![Все дьяволы здесь | All the Devils are Here (2025)]() 1:31:39
1:31:39
![Код 3 | Code 3 (2025)]() 1:39:56
1:39:56
![Диспетчер | Relay (2025)]() 1:51:56
1:51:56
![Отчаянный | Desperado (1995) (Гоблин)]() 1:40:18
1:40:18
![Карты, деньги, два ствола | Lock, Stock and Two Smoking Barrels (1998) (Гоблин)]() 1:47:27
1:47:27
![Дом из динамита | A House of Dynamite (2025)]() 1:55:08
1:55:08
![Гедда | Hedda (2025)]() 1:48:23
1:48:23
![Грязь | Filth (2013) (Гоблин)]() 1:37:25
1:37:25
![Рок-н-рольщик | RocknRolla (2008) (Гоблин)]() 1:54:23
1:54:23
![Трон: Арес | Tron: Ares (2025)]() 1:52:27
1:52:27
![Заклятие 4: Последний обряд | The Conjuring: Last Rites (2025)]() 2:15:54
2:15:54
![Чумовая пятница 2 | Freakier Friday (2025)]() 1:50:38
1:50:38
![Терминатор 2: Судный день | Terminator 2: Judgment Day (1991) (Гоблин)]() 2:36:13
2:36:13
![Свинтусы | The Twits (2025)]() 1:42:50
1:42:50
![Орудия | Weapons (2025)]() 2:08:34
2:08:34
![Плохой Санта 2 | Bad Santa 2 (2016) (Гоблин)]() 1:34:55
1:34:55
![Мужчина у меня в подвале | The Man in My Basement (2025)]() 1:54:48
1:54:48
![Святые из Бундока | The Boondock Saints (1999) (Гоблин)]() 1:48:30
1:48:30
![Тайны Медовой долины]() 7:01
7:01
![Корги по имени Моко. Домашние животные]() 1:13
1:13
![Космический рейнджер Роджер Сезон 1]() 11:32
11:32
![Мотофайтеры]() 13:10
13:10
![Полли Покет Сезон 1]() 21:30
21:30
![Пиратская школа]() 11:06
11:06
![Оранжевая корова]() 6:30
6:30
![Зебра в клеточку]() 6:30
6:30
![Простоквашино. Финансовая грамотность]() 3:27
3:27
![Тёплая анимация | Новая авторская анимация Союзмультфильма]() 10:21
10:21
![Крутиксы]() 11:00
11:00
![Команда Дино Сезон 1]() 12:08
12:08
![Истории Баданаму Сезон 1]() 10:02
10:02
![Команда Дино. Исследователи Сезон 1]() 13:10
13:10
![Сборники «Оранжевая корова»]() 1:05:15
1:05:15
![Псэмми. Пять детей и волшебство Сезон 1]() 12:17
12:17
![Супер Зак]() 11:38
11:38
![Забавные медвежата]() 13:00
13:00
![Папа Супергерой Сезон 1]() 4:28
4:28
![Пакман в мире привидений]() 21:37
21:37

 4:18
4:18Скачать видео
| 256x144 | ||
| 640x360 | ||
| 1280x720 |
 2:34
2:34
2025-11-05 11:45
 3:33
3:33
2025-11-07 13:31
 3:33
3:33
2025-11-02 10:06
 3:36
3:36
2025-11-02 10:25
 2:49
2:49
2025-11-04 17:50
 3:33
3:33
2025-11-02 10:17
 3:53
3:53
2025-11-06 12:59
 3:25
3:25
2025-11-02 10:34
 2:29
2:29
2025-11-07 13:53
 4:10
4:10
2025-11-06 11:31
 2:49
2:49
2025-11-06 13:20
 3:20
3:20
2025-11-07 13:34
 3:00
3:00
2025-11-03 12:33
 3:18
3:18
2025-11-02 10:30
 5:08
5:08
2025-10-31 12:50
 3:24
3:24
2025-11-07 14:37
 3:40
3:40
2025-11-05 00:22
 3:17
3:17
2025-11-06 12:00
 3:10
3:10
2025-11-07 13:57
 3:41
3:41
2025-11-05 11:55
0/0
 1:23:01
1:23:01
2025-11-05 19:47
 2:03:12
2:03:12
2025-11-07 20:11
 1:31:39
1:31:39
2025-10-02 20:46
 1:39:56
1:39:56
2025-10-02 20:46
 1:51:56
1:51:56
2025-09-24 11:35
 1:40:18
1:40:18
2025-09-23 22:53
 1:47:27
1:47:27
2025-09-23 22:52
 1:55:08
1:55:08
2025-10-29 16:30
 1:48:23
1:48:23
2025-11-05 19:47
 1:37:25
1:37:25
2025-09-23 22:52
 1:54:23
1:54:23
2025-09-23 22:53
 1:52:27
1:52:27
2025-11-06 18:12
 2:15:54
2:15:54
2025-10-13 19:02
 1:50:38
1:50:38
2025-10-16 16:08
 2:36:13
2:36:13
2025-10-07 09:27
 1:42:50
1:42:50
2025-10-21 16:19
 2:08:34
2:08:34
2025-09-24 22:05
 1:34:55
1:34:55
2025-09-23 22:53
 1:54:48
1:54:48
2025-10-01 15:17
 1:48:30
1:48:30
2025-09-23 22:53
0/0
 7:01
7:01
2022-03-30 17:25
 1:13
1:13
2024-11-29 14:40
2021-09-22 21:49
 13:10
13:10
2024-11-27 14:57
2021-09-22 23:09
 11:06
11:06
2022-04-01 15:56
 6:30
6:30
2022-03-31 18:49
 6:30
6:30
2022-03-31 13:09
 3:27
3:27
2024-12-07 11:00
 10:21
10:21
2025-09-11 10:05
 11:00
11:00
2022-07-25 18:59
2021-09-22 22:29
2021-09-22 21:29
2021-09-22 22:45
 1:05:15
1:05:15
2025-09-30 13:45
2021-09-22 22:23
2021-09-22 22:07
 13:00
13:00
2024-12-02 13:15
2021-09-22 21:52
 21:37
21:37
2024-11-28 17:35
0/0

